TransMedics keeps donor hearts beating en route to transplant patients
In 1967, doctors in a South African hospital performed the very first successful human heart transplant. Now approximately 5,000 heart transplants are performed worldwide each year. At 2,000 transplants a year, U.S. surgeons handle the the bulk of those procedures. Heart surgery has become more efficient and fairly routine over time, but one aspect of the organ transplant process hasn't changed since that first operation:

Transport.
Delivering donor organs is always a race against time. Organs are still placed on ice in plastic coolers, and the rush to move them to hospitals in need looks a lot like it does on movies and television. Today, eight out of 10 hearts never reach patients who need them due to numerous complications. One major complication is time. A heart on ice is only viable for approximately six hours. The greater the distance between hospitals, the great the risk. By the time donor hearts reach potential recipients, the coolers have often damaged the donor hearts too much to use successfully.
Enter the Organ Care System (OCS) developed by TransMedics.
The Ronald Reagan UCLA Medical Center has been testing the machines for use with donor hearts since 2010. This new, revolutionary device pumps blood through human hearts, allowing them to stay warm and survive longer during transport.
"TechKnow" contributor Phil Torres visits UCLA to see the OCS first-hand.
"A human organ has never been kept alive outside of a human body until this machine became a clinical reality," says Dr. Abbas Ardehali, the head of UCLA's heart and lung transplant program. He's a cardiothoracic surgeon who has worked closely with the OCS for the last couple years. "It makes intuitive sense to a layperson to say, 'Instead of having my heart on ice, I want it to be warm. I want it to be beating.'"
For Sandra Aguilar, a UCLA patient who was on the waiting list for a heart when this episode was shot, the organ care technology is a possible second chance at life. Suffering from cardiomyopathy, Aguilar had been forced to stop working or do much of anything.
"I can't spend time with my kids, I can't go out," she tells Phil in Spanish. She decided to participate in UCLA's program to help their research -- testing the outcomes of patients who receive hearts using the OCS versus those who don't.
UCLA and TransMedics believe that the OCS can greatly improve these numbers, increasing the delivery window from six to 12 hours. OCS also allows doctors to monitor the heart as it travels, helping to guarantee that the organ remains at optimal condition right up to the transplant procedure.
According to Dr. Waleed Hassanein, the founder and president of TransMedics, "We feel very comfortable and confident in the Organ Care System's ability to at least double the heart transplant volume over the next 5 years."
He hopes that the OCS will eventually become part of the standard training for surgeons. Until then, the majority of doctors who have used the OCS to practice monitoring and moving pig hearts instead of human hearts as the two are very similar anatomically. Phil was able to watch a doctor connect a pig heart to the OCS through the aorta to see exactly how the organ goes from still to beating again in a matter of moments.
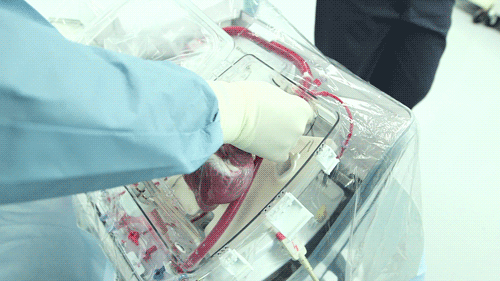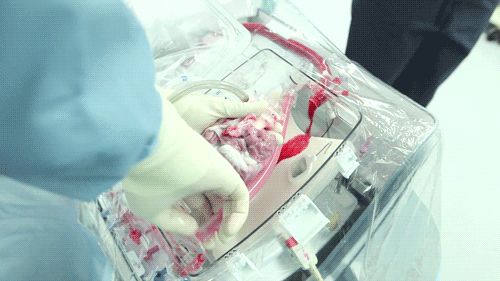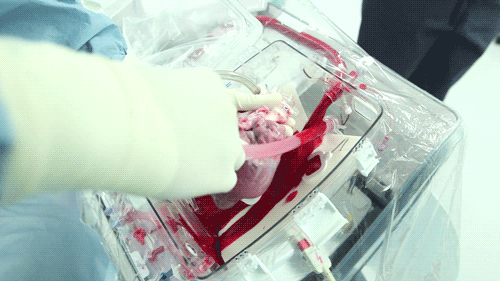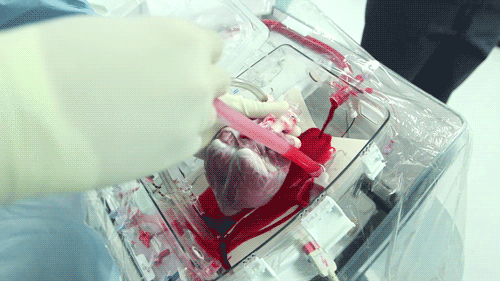
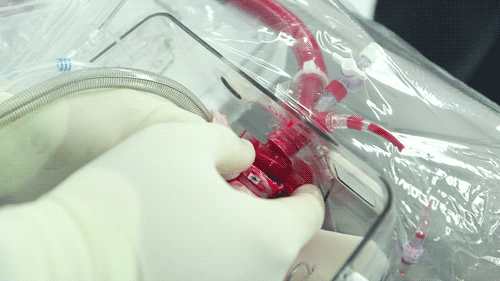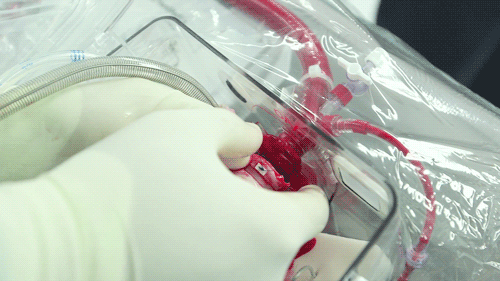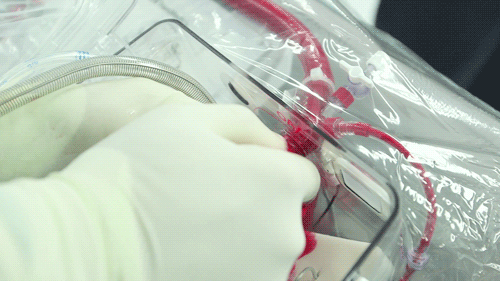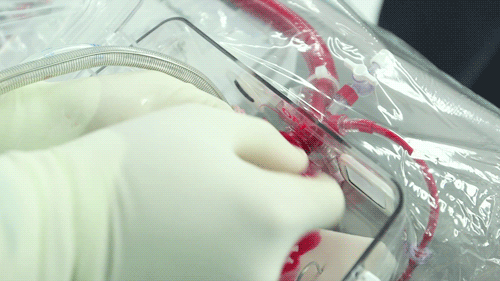
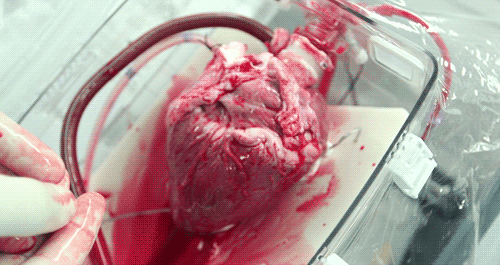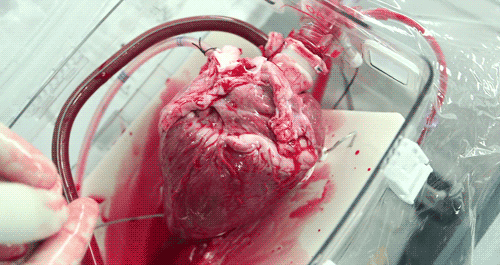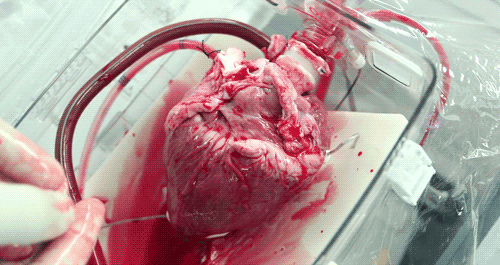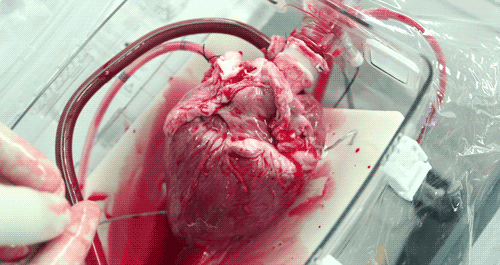
The OCS uses the donor's own warm blood and a formula of nutrients prepared by doctors. If a hospital has access to enough fresh blood, the OCS can theoretically keep the heart alive indefinitely.
TransMedics hopes to develop their technology to care for a variety of human organs. A similar study has been conducted with human lungs. In 2012, UCLA Medical Center successfully completed the first "breathing lung" transplant in the U.S.

The company has also tested treating infected organs outside of the body and then reintroducing disease-free organs back into patients to potentially spare patients from drawn-out, unnecessarily painful treatments for conditions such as pneumonia or -- maybe one day -- cancer.
In the last two years, the OCS has already improved the survival rate of transplant patients by 30 percent. Eight countries outside of the U.S. have hospitals using the OCS, and TransMedics hopes that the Food and Drug Administration (FDA) will approve use of the OCS beyond clinical study within the United States in 2014.
"It may sound like science fiction," Phil says, "but when you see this machine work, you begin to believe it."
Watch "TechKnow," Sundays at 7:30PM ET/4:30PM PT on Al Jazeera America.
Error
Sorry, your comment was not saved due to a technical problem. Please try again later or using a different browser.